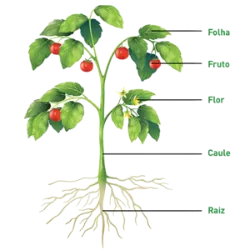
Specific heat
History of Moderator - Lisboa, Portugal

Specific heat is an intensive physical quantity that describes the thermal variation of a substance when it receives a certain amount of heat. Also known as mass heat capacity, it is measured in units such as J/(kg.K) or cal/(g °C). Specific heat is calculated using the formula c = Q/m. ΔT or c = C/m. In addition, the molar specific heat, also called the molar heat capacity, is calculated by the relationship between the heat capacity and the number of moles present. Latent heat, in turn, is the amount of heat received or given off by a body, where its temperature remains the same, while its physical state changes. This quantity is measured by the calculation C = Q/ΔT... Did you know?